Research
Polymer Chemistry with an Inorganic Touch !
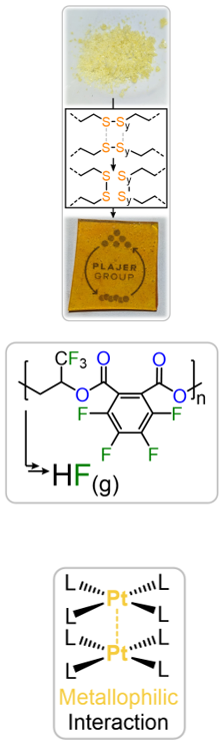
Sulfur containing polymers: Sulfur-containing polymers can exhibit improved semi-crystallinity, recyclability and degradability in comparison to their all-oxygen-containing counterparts, as well as unique properties such as high refractive indices rendering them, for example, as useful components in optical applications. In this regard the work in our group concerns ring-opening copolymerization of a strained heterocycle such as epoxides and oxetanes with sulfur containing comonomers such as carbon disulfide, thioanhydrides and even elemental sulfur itself, a waste product of the petrochemical industry. Other than completely turning catalysis upside down compared to traditional ring-opening polymerisation the resulting materials allow for exciting materials applications such as in dynamic covalent networks or crystallisation driven self-assembly.
Fluorine containing polymers: Fluorinated polymers are not only popular materials in a wide range of consumer applications but are currently irreplaceable in many industries. The low polarizability of the fluorine groups, for example, renders these polymers more hydrophobic and less adhesive than their non-fluorinated counterparts, making them useful as water repellents and low-friction surface coatings. Unfortunately, these materials have come under much scrutiny for being “forever chemicals,” meaning they do not appreciably degrade in the environment over time. As part of the CRC 1349, we succeeded in synthesizing degradable fluorinated polyesters that exhibit the classic benefits of fluorinated polymers but remain degradable. Furthermore, they enable chemical recycling methods to extract and recover the fluorine into a useful form once again.
Transition metal containing polymers: Non-covalent interactions, such as hydrogen bonding or π-π interactions, confer the most extraordinary properties to polymer materials. However, a scarcely explored interaction in macromolecular chemistry is the metallophilic interaction. Here, complexes of late transition metals (mostly Pt, Au, or Hg) are attracted to each other by relativistically enhanced van der Waals forces. A new line of research in our group will hence focus on exploiting metallophilic interactions to construct organic-inorganic hybrid materials in which metal chains interconnect polymer chains. Employing intrinsically chiral polymers might allow for the self-assembly of chiral superstructures for the next generation of chiral electronics, biological sensors, or target-specific photodynamic therapeutics. Furthermore, dynamic covalent ligand sets might enable their deconstruction to aid chemical recyclability.


